Maintenance of Energy Metabolism Is an Integral Part of Plakophilin-2 and Desmosome Functions
Authors: J. Alex Aycinena, Anley E. Tefera, Isaac Perea-Gil, Duncan Holbrook-Smith, Kevin Williams, Reva Shenwai, Farshad Farshidfar, Brendan Ryback, Katelyn Foppe, Iris Wu, Aliya Zeng, Melissa Van Pell, Emma Xu, Joseph Woods, Samantha Jones, Yolanda Hatter, Cristina Dee-Hoskins, Jessie Madariaga, Kevin Robinson, Amara Greer-Short, Xiaomei Song, Kathryn N. Ivey, James Priest, Gretchen Argast, Timothy Hoey, Edward Driggers, and Zhihong Jane Yang
Originally Published in: JACC: Basic to Translational Science (December 2025)
Summary
Breaking New Ground in ARVC Research: Linking Cardiac Structure, Function, and Metabolism in 3D
One of the biggest challenges in building predictive in vitro cardiac models is recapitulating the mechanical and metabolic context the heart needs to function. This is especially challenging for arrhythmogenic right ventricular cardiomyopathy (ARVC), a familial disease that can cause arrhythmias and sudden cardiac death in response to cardiac remodeling and mechanical instability.
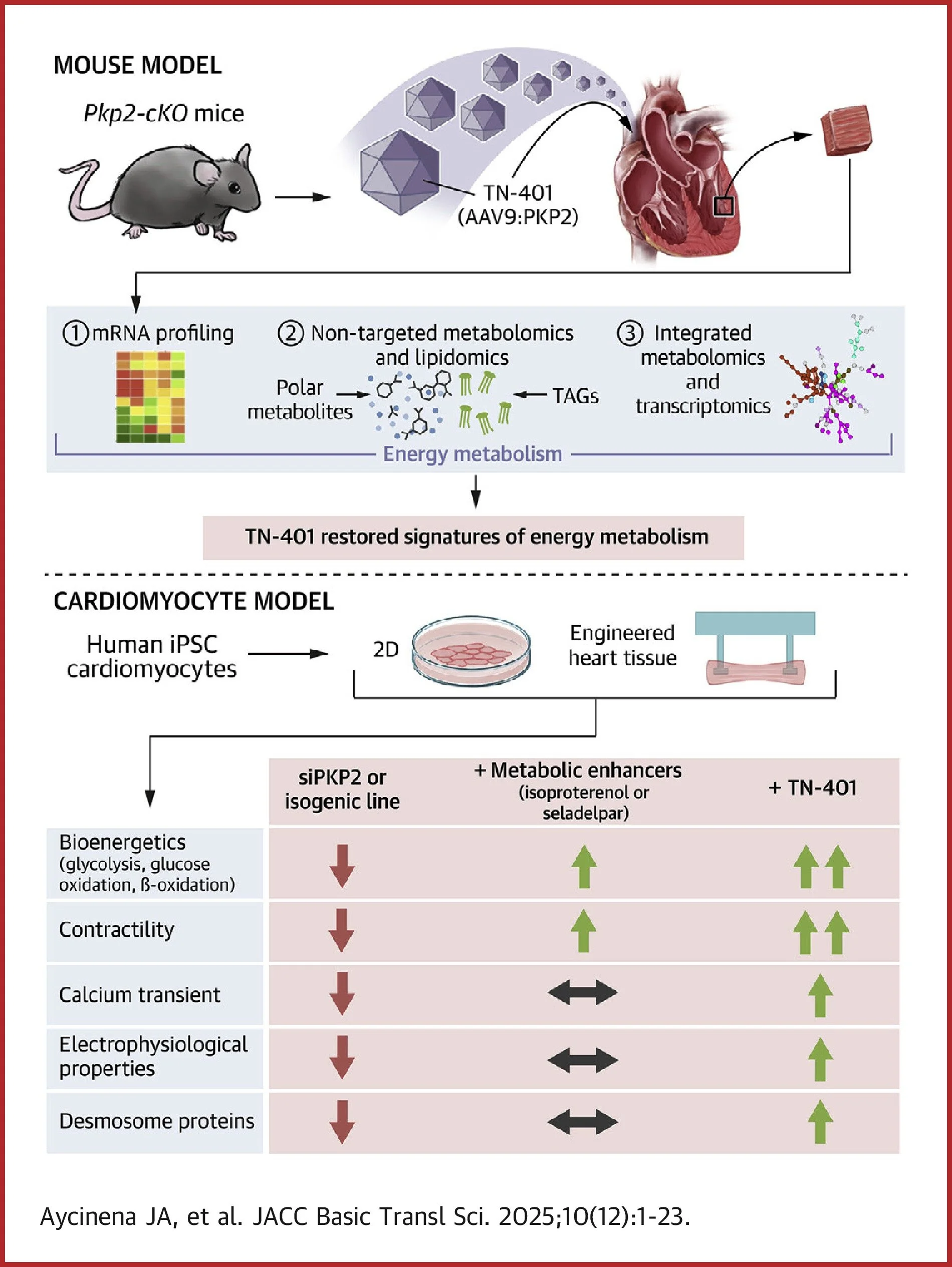
In the new JACC: Basic to Translational Science publication, "Maintenance of Energy Metabolism Is an Integral Part of Plakophilin-2 (PKP2) and Desmosome Functions," researchers uncover a fundamental link between cardiac structure and energy metabolism. Using human hiPSC-derived engineered heart tissues (EHTs) and a Pkp2-cKO mouse model, the teams from Tenaya Therapeutics, General Metabolics, and UCLA Lipidomics demonstrate that loss of PKP2 disrupts both desmosomal integrity and metabolic homeostasis, including impaired fatty acid oxidation and glycolysis. By pairing PKP2-deficient 3D EHTs with Curi Bio’s Mantarray™, Nautilai™, and Pulse™ platforms, the authors integrated metabolic flux, calcium handling, and contraction force measurements to translate PKP2-driven molecular dysfunction into quantitative, real-time readouts of cardiac structural and functional decay.
Key insights include regarding their use of Curi Bio technologies:
Mantarray was used to measure forces in PKP2-deficient 3D EHTs, showing a 65% reduction in twitch force with 50% slowing in contraction and relaxation velocities, mirroring the cardiac dysfunction observed in vivo.
Using Curi Bio Nautilai optical mapping, the authors identified PKP2-dependent calcium defects in both 2D cardiomyocyte monolayers and 3D EHTs, including slowed calcium rise, prolonged transients, and impaired excitation–contraction coupling.
Treatment with TN-401 (AAV9:PKP2) demonstrates cross-model concordance, extending median mouse lifespan by over 50 weeks and restoring twitch force and kinetics in human 3D EHTs to wild-type levels.
These results reinforce a powerful concept: cardiac structure and metabolism are inseparable determinants of function. Beyond these compelling results, this study also illustrates a roadmap for using Curi Bio’s 3D EHT NAM ecosystem that can meet or transcend animal research and bridge the translational gap between the bench and the patient, unlocking the human-relevant biology needed to solve the heart's complex cardiovascular pathologies.
Congratulations to the entire team on this excellent publication!