Functionally Mature Bioengineered Human Skeletal Muscle Tissues Capture Essential Aspects of Glucose Metabolism
Authors: Carlos Henriquez-Olguin, Martina Kubec Højfeldt, Christopher Thomas Andrew Lewis, Jesper B. Birk, Tianfang Wang, Zelin Li, Pia Jensen, Pauline Blomquist, Bo Falck Hansen, Roberto Meneses-Valdes, Enrique Toledo, Jeb Hogan, Zhe Wang, Charis-Patricia Segeritz, Jørgen Frank Pind Wojtaszewski, Thomas Elbenhardt Jensen, Anna Blois, Christian Pehmøller, Jonas Roland Knudsen
Originally Published in: BioRxiv (January 2026) (Download the Preprint)
Summary
Engineering Human Skeletal Muscle to Advance Translational Metabolic Research
A major challenge in metabolic drug development has been the lack of in vitro models that faithfully recapitulate the structural and metabolic maturity of adult human tissue. Animal models often fail to be clinically predictive due to fundamental interspecies differences in fiber composition and glycogen storage.
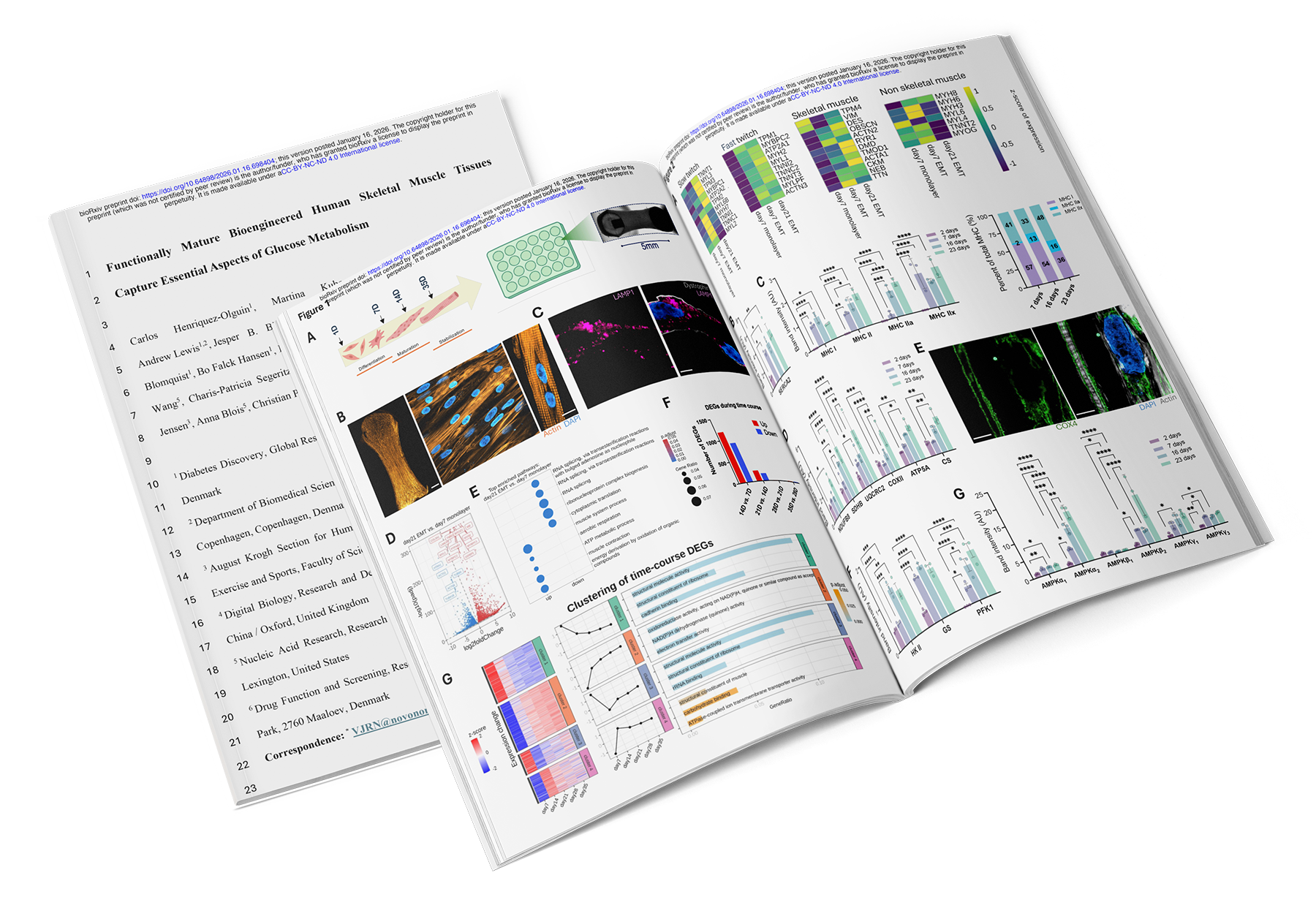
In a new bioRxiv preprint, "Functionally Mature Bioengineered Human Skeletal Muscle Tissues Capture Essential Aspects of Glucose Metabolism," researchers from Novo Nordisk and the University of Copenhagen present a comprehensive workflow for generating 3D bioengineered human skeletal muscle tissues that mature into a stable, adult-like metabolic state.
Using the Mantarray™ 3D skeletal muscle ecosystem and optimized skeletal muscle media from Curi Bio, the team integrated physiological, pharmacological, and genetic perturbations with transcriptomic and protein assays to interrogate mechanisms underlying metabolic health and activity-dependent muscle plasticity.
Key Breakthroughs from this Study Include
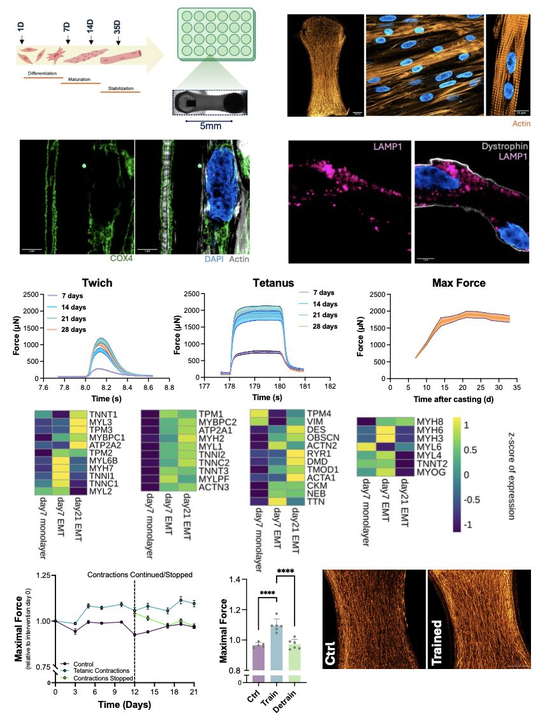
Exercise physiology in vitro: Exercise-mimicking stimulation increases force and endurance, with fully reversible gains upon detraining, closely mirroring human muscle plasticity in vivo.
Predictive metabolic function: Tissues exhibited robust insulin-stimulated glucose uptake and punctate GLUT4 localization throughout the myotubes, a major milestone for predictive diabetes and obesity research.
Functional stability: Tissues reached a stable plateau by day 21 and remained contractile for over a month, enabling longitudinal and mechanistic studies.
New biology uncovered: The study revealed a functional role for GYS1 in sustaining muscle endurance under metabolic stress, linking glycogen metabolism directly to performance.
These findings provide a clear roadmap for how new approach methodologies (NAMs) can overcome long-standing translational barriers by delivering predictive, human-relevant functional data.
👏 Congratulations to the teams at Novo Nordisk and the University of Copenhagen on this landmark contribution to muscle modeling and metabolic research.