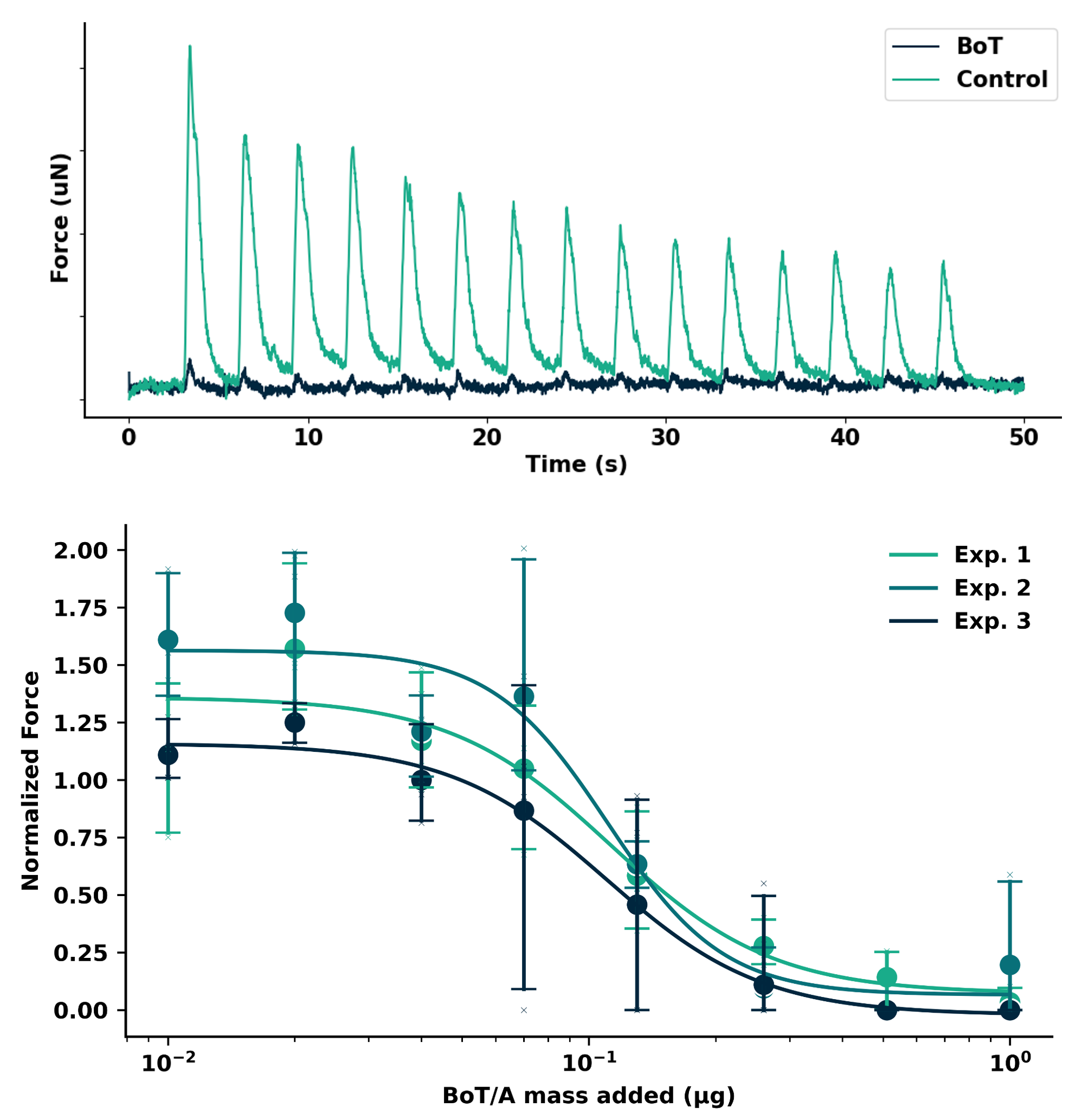
3D Human Neuromuscular Junction Model
Scalable, Functional & Quantifiable
A high-throughput, iPSC-derived platform delivering unparalleled reproducibility for neurotoxin testing, drug discovery and disease modeling.
Histological Characterization of NMJs